The World Health Organization decides BA.2 is more transmissible than BA.1
https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—8-march-2022
https://www.nytimes.com/interactive/2022/03/28/opinion/coronavirus-mutation-future.html
Weekly epidemiological update on COVID-19
BA.2 is main global driver
Feb. 16th to March 17th
BA.2 86% of global reports
BA.1 and BA.1.1, together 13%
BA.2 is more transmissible than BA.1
Not been shown to cause more severe illness
Dr. Maria Van Kerkhove
Our vaccines remain incredibly effective at preventing severe disease and death, including against both of the sublineages of BA.1 and BA.2
Deltacron
Shanghai
https://www.theguardian.com/world/2022/mar/28/shanghai-to-lock-down-millions-for-mass-testing-as-chinas-covid-cases-surge
Phased lockdown, 25 million people
Omicron wave
Eastern side, under lockdown, Monday to Friday
Mass testing
Cases, 3,450 asymptomatic cases
(50 symptomatic cases)
Other citywide lockdowns
Vaccine effectiveness of two and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong
https://www.medrxiv.org/content/10.1101/2022.03.22.22272769v1
Three doses of either vaccine offered very high levels of protection against severe outcomes (VE: 98.1%)
Third doses of either BNT162b2 or CoronaVac provide substantial additional protection against severe COVID-19 and should be prioritized,
particularly in older adults who received CoronaVac primary schedules.
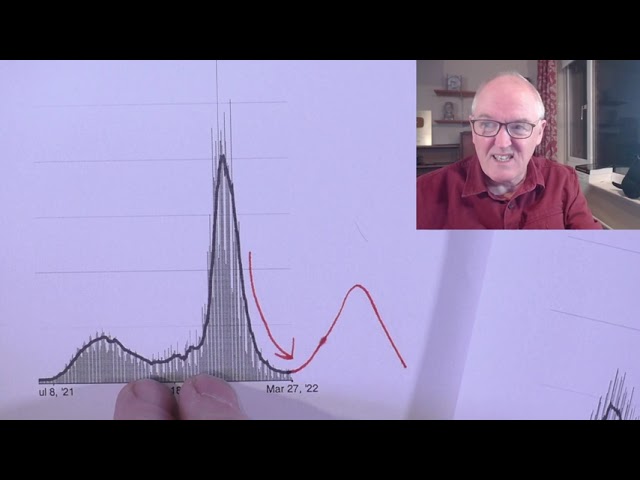
US
Dr. Rochelle Walensky, BA.2, third of new cases
Paxlovid
US, cases
https://covid.cdc.gov/covid-data-tracker/#datatracker-home
US deaths
US, hospital patients
https://covid19.healthdata.org/united-states-of-america?view=cumulative-deaths&tab=trend
A second booster for anyone 50 and older is expected soon (FDA, CDC)
Four months after thirds shot
Source